

























Ionic cleanliness usually refers to the ionic cleanliness of printed circuit boards. Since various materials of printed circuit boards cause ionic residues during the cleaning process, it will affect the functionality and reliability of electronic products.
| Project Overview
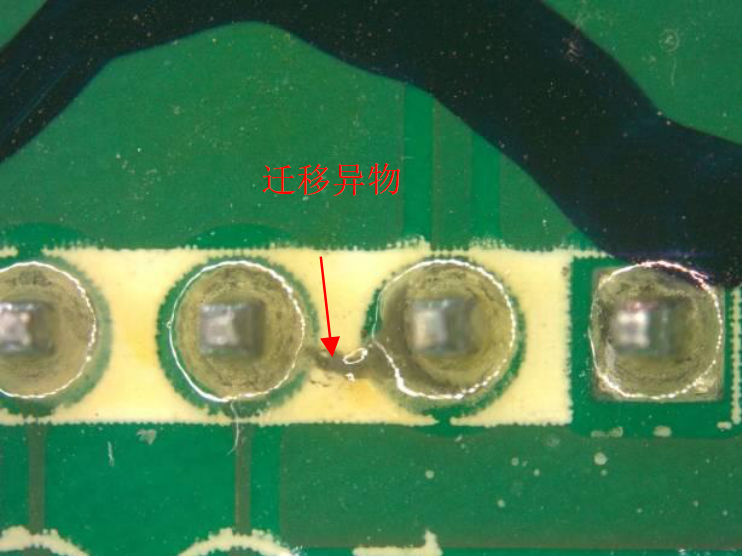
Ionic cleanliness usually refers to the ionic cleanliness of printed circuit boards. Since various materials of printed circuit boards cause ionic residues during the cleaning process, it will affect the functionality and reliability of electronic products. The most common problems caused by ionic contamination are surface corrosion and crystal growth, which ultimately lead to short circuits. Excessive current passing through the connectors causes the final damage to electronic products.
Several types of pollutants mainly come from processes such as electroplating, wave soldering, reflow soldering, and chemical cleaning. There are mainly five forms of pollution, including flux residues, ionized surface activators, ethanol, amino ethanol, and human sweat.
| Testing Standards
IPC TM-650 2.3.28.2 Bare Printed Board Cleanliness by Ion Chromatography
IPC TM-650 2.3.28B Ionic Analysis of Circuit Boards, Ion Chromatography Method
| Main Types of Ions to Be Tested:
|
阴离子 Anion |
阳离子 Cations |
有机酸 Organic acids |
|
氟离子(F -) Fluoride ion (F -) |
锂离子(Li+) Lithium ion (Li+) |
乙酸(CH3COOH) Acetic acid (CH3COOH) |
|
氯离子(Cl -) Chloride ion (Cl -) |
钠离子(Na+) Sodium ion (Na+) |
甲酸(CH2O2) Formic acid (CH2O2) |
|
亚硝酸根离子(NO2-) Nitrite ion (NO2-) |
铵根离子(NH4+) Ammonium ion (NH4+) |
甲基磺酸(CH4O3S) Methanesulfonic acid (CH4O3S) |
|
硫酸根离子(SO4 2-) Sulfate ion (SO4 2-) |
钾离子(K+) Potassium ion (K+) |
己二酸(C6H10O4) Adipic acid (C6H10O4) |
|
溴离子(Br -) Bromide ion (Br-) |
镁离子(Mg2+) Magnesium ion (Mg2+) |
琥珀酸(C4H6O4) Succinic acid (C4H6O4) |
|
硝酸根离子(NO3- ) Nitrate ion (NO3- ) |
钙离子(Ca2+) Calcium ion (Ca2+) |
苹果酸(C4H6O5) Malic acid (C4H6O5) |
|
磷酸根离子(PO4 3-) Phosphate ion (PO4 3-) |
/ |
邻苯二甲酸(C8H6O4) Phthalic acid (C8H6O4) |
|
/ |
/ |
谷氨酸(C5H9NO4) Glutamic acid (C5H9NO4) |
| Testing Methods
1 Cation-anion testing method
The cation-anion method is used to test the ionic cleanliness. It mainly involves soaking the surface of the sample in a mixed solution of isopropyl alcohol and water in a certain proportion to dissolve the contaminants on the sample surface into the solution. Then, the pollution degree is analyzed by testing the types and contents of cations, anions and organic acids in the solution. The test results are generally expressed in ug/cm². The test standard refers to IPC-TM-650, 2, 3, 28 The advantage of this method is that it can provide detailed information on the residual types and contents of various ions. The IPC-TM-650, 2, 3, 28 standard stipulates that the testing items of the cation-anion method mainly include 6 cations, 7 anions and 8 organic acids.
Namely:
|
阳离子 Cations |
锂离子、钠离子、铵根离子、钾离子、镁离子、钙离子 Lithium ion, sodium ion, ammonium ion, potassium ion, magnesium ion, calcium ion |
|
阴离子 Anion |
氟离子、氯离子、亚硝酸根离子、溴离子、硝酸根、磷酸根、硫酸根 Fluoride ion, chloride ion, nitrite ion, bromide ion, nitrate ion, phosphate ion, sulfate ion |
|
有机酸 Organic acids |
乙酸、己二酸、甲酸、谷氨酸、苹果酸、甲烷磺酸盐、琥铂酸、邻苯二甲酸 Acetic acid, adipic acid, formic acid, glutamic acid, malic acid, methanesulfonate, succinic acid, phthalic acid |
2 Sodium chloride equivalent method
The sodium chloride equivalent method uses an ultra-pure extraction solution to remove the residues left in the process from electronic components. The measurement of the cleaning result is based on conductivity or resistivity as the evaluation criteria.
In the test, a solution prepared with pure 75% IPA (isopropyl alcohol) and 25% deionized water (by volume) is used as the extraction solution. Place the PCB in the extraction solution, and measure the conductivity or resistivity of the extraction solution after the pollutants in the PCB are dissolved, which serves as the basis for the pollutants. Due to the different types of ions in the pollutants, in the test, the ion concentration of equivalent NaCl is uniformly used to represent them, that is, expressed in μgEq NaCl/cm². The magnitude of the ion concentration of equivalent NaCl is used as the parameter for the degree of ionic contamination. Its reference standard is IPC-TM-650, 2, 3, 25C The disadvantage of this method compared with the anion-cation method is that this method cannot clearly know the types and contents of ionic contaminants.
| How to Choose the Testing Method?
Generally, if you want to clearly understand the types and contents of pollutants, it is recommended to choose the IPC-TM-650, 2, 3, 28 anion-cation method. This method can clearly understand the sources of pollutants.
Example:
If the contents of F, Cl, and Br in the test results are relatively high, it can be judged that the source of pollution may be caused by the residues of solder resists in the wave soldering and reflow soldering processes; if the results of organic acids in the test results are relatively high, it may be the pollution introduced during the cleaning process with ethanol or aminoethanol. The sodium chloride equivalent method does not have this advantage.
| MTT Advantages
1. Professional Team: Equipped with a number of highly experienced testing engineers and technical experts.
2. Advanced Equipment: Equipped with internationally leading testing instruments to ensure accuracy and reliability of results.
3. Efficient Service: Rapidly respond to customer needs and provide one-stop, high-efficiency inspection services.
4. Authoritative Certification: The laboratory is certified by ISO/IEC 17025, ensuring that test reports have international credibility.